 |
Rapid evolution, diversification and distribution
of mosasaurs (Reptilia; Squamata) prior to the K-T boundary
Michael J. Everhart
Sternberg Museum of Natural History, Fort Hays State University, Hays, Kansas, 67601.
Webpage created 12/03/2005; updated 06/11/2010
Copyright © 2005-2010 by Mike Everhart
LEFT: Here a Late Cretaceous Mosasaurus
hobetsuensis Suzuki (1985) cruises along the rocky shores of what would become modern
day Japan. Copyright © 2002 by Dan Varner; used with permission of Dan
Varner. This picture was painted for me as a gift for my host at the Morphogenesis
conference I attended in Nagoya, Japan in 2002. |
PLEASE NOTE: This web page is the result of
two earlier papers I presented at conferences: The International Conference on
Morphogenesis and Pattern Formation in Biological Systems, Nagoya, Japan, September 24-27,
2002, and the Tate Museum 11th Annual Symposium (June, 2005), in Casper, Wyoming.
Neither paper was peer-reviewed and this webpage should not
be cited as a reference:
Everhart, M. J., 2002. Rapid ontogenetic change in Late Cretaceous mosasaurs (Reptilia;
Mosasauridae) as a model of vertebrate morphogenesis. International Conference on
Morphogenesis and Pattern Formation in Biological Systems, Chubu University, Nagoya,
Japan, p. 86. Everhart, M. J. 2005. Rapid
evolution, diversification and distribution of mosasaurs (Reptilia; Squamata) prior to the
K-T Boundary. Tate 2005 11th Annual Symposium in Paleontology and Geology, Casper, WY, p.
16-27 |
Rapid evolution, diversification and distribution
of mosasaurs (Reptilia; Squamata) prior to the K-T boundary
Michael J. Everhart
Sternberg Museum of Natural History, Fort Hays State University,
Hays, Kansas, 67601.
ABSTRACT
Highly-adapted marine reptiles called mosasaurs became the apex predators of the
Earth’s oceans in the last 25 million years of the Late Cretaceous. Returning to the
sea during Cenomanian - Turonian time, they evolved from 1 m, shore-dwelling lizards into
a variety of large (up to 17 m), fully marine carnivores. Their remains have been found in
marine deposits on all continents, including Antarctica. Evidence of their rapid
radiation, worldwide distribution and dominance appears to contrast sharply with that of
other marine reptiles such as ichthyosaurs and plesiosaurs, the dinosaurs and even other
marine predators such as sharks. Mosasaurs were highly adaptable and apparently able to
fill many of the ecological niches left vacant by the extinction of the ichthyosaurs,
marine crocodiles and plesiosaurs. Their rapid evolution may have also contributed to the
extinction of several species of sharks, and they may have been competing with
crocodilians in estuarine and freshwater environments at the end of the Cretaceous.
Mosasaurs are an example of adaptive radiation prior to the K-T boundary extinction.
INTRODUCTION
Following the extinction event(s) near the end of the Permian, the evolution and
diversification of reptiles produced a great variety of extinct and modern forms including
dinosaurs, birds, pterosaurs, lizards, turtles, snakes, and a diverse group that is
generally referred to as marine reptiles. During the Triassic, some marine reptiles,
including ichthyosaurs and plesiosaurs, evolved into a number of successful families that
persisted through most of the Mesozoic. By the end of the Early Cretaceous, however,
Ichthyosaurs had become extinct. During the Turonian, plesiosaurs were reduced to two
families, the elasmosaurids and polycotylids, which apparently survived into the
Maastrichtian. Mosasaurs were the last major group of reptiles to return to the sea,
evolving from at least two (Russell, 1967), or possibly three or more (Bell and Polcyn,
2004) lineages of small, terrestrial lizards called aigialosaurs. They diversified
rapidly, spread quickly and flourished in the Earth’s oceans during the last 25
million years of the Late Cretaceous. Their rapid rise and dominance occurred over a much
shorter period of time than other marine reptiles and sharks, or terrestrial groups such
as dinosaurs and mammals.
The remains of mosasaurs were initially collected in the Netherlands in the mid-1700s,
some fifty years before the discovery of dinosaurs, and were among the first recognized
fossils of large animals. Adrian Camper (1800) is credited as the first to recognize the
relationship between the remains of “Le Grand Animal of
Maastricht” (Mosasaurus hoffmanni)
and varanid lizards (e. g., Komodo dragon). By 1850,
mosasaur remains had been found in Cretaceous marine deposits around the world, including
England, New Jersey, South Dakota and New Zealand. The 1868
discovery of the type specimen of Tylosaurus proriger in western Kansas
brought two well known paleontologists of the day (O. C. Marsh and E. D. Cope) to the
state, and resulted in the collection of literally thousands of specimens from the Smoky Hill Chalk over an interval of about ten years. Since that
time, mosasaur remains have been discovered on every continent, including Antarctica, and
new species are being described and named at a steady pace (Table 1).
Table 1 –
New species of mosasaurs described 1985-2008. Arranged by time of occurrence. |
Halisaurus arambourgi
Prognathodon
kianda
Prognathodon saturator
Mosasaurus
hobetsuensis
Lakumasaurus
antarcticus
Mosasaurus
prismaticus
Pluridens
walkeri
Selmasaurus
russelli
Prognathodon
currii
Globidens
schurmanni
Prognathodon
stadtmani
Kourisodon
puntledgensis
Selmasaurus
johnsoni
Tylosaurus
kansasensis
Yaguarasaurus
columbianus
Dallasaurus
turneri
Russellosaurus
coheni
Tethysaurus
nopscai
Haasiasaurus
gittelmani |
New
species of Halisaurus described from the upper Maastrichtian of Morocco, Africa,
by Bardet, et al. (2005).
New species of Prognathodon described from the upper Maastrichtian of Angola, Africa, by
Schulp, et al. (2008)
New species of Prognathodon described from the upper Maastrichtian
of the Netherlands, northern Europe, by Dortangs, et al., (2002).
New species of mosasaurine described from the lower Maastrichtian of Hokkaido, Japan by
Suzuki, 1985b.
New
tylosaurine described from the upper Campanian / lower Maastrichtian of Antarctica by
Novas, et al, (2002)
New mosasaurine described from the upper Campanian / lower Maastrichtian of Hokkaido,
Japan by Sakurai and Shibuya, 1999.
New genus and species from the Upper Campanian / Lower Maastrichtian of Niger, Africa,
described by Lingham-Soliar (1998)
New plioplatecarpine from the Upper Campanian / Lower Maastrichtian of Alabama, North
America, by Wright and Shannon (1988)
New species of Prognathodon from the upper Campanian of Israel described by
Christiansen and Bonde (2002)
New species of Globidens from the upper Campanian of South Dakota, North America,
reported by Martin 2007.
New species of Prognathodon from the lower Campanian of Utah, North America,
described by Kass (1999).
New mosasaur from the upper Santonian of Vancouver Island, North America, described by
Nicholls and Meckert, 2002.
New plioplatecarpine from the Lower Santonian of Kansas, North America, described by
Polcyn and Everhart (2008)
New
tylosaurine from the upper Coniacian of Kansas, North America, described by Everhart
(2005a).
New mosasauroid genus and species from the Turonian of Columbia, South America, by
Páramo-Fonseca (2000).
New mosasauroid from the middle Turonian of Texas, North America, by Bell and Polcyn
(2005)
New mosasauroid genus and species from the middle Turonian of Texas, North America, by
Polcyn and Bell, (2005).
New mosasauroid genus and species from the lower Turonian of Morocco, North Africa, by
Bardet, et al. (2003).
New mosasauroid genus and species from the lower Cenomanian of Israel by Polcyn, et al.
(1999; 2003). |
By the end of the Cretaceous, two genera of mosasaurs (Mosasaurus and Hainosaurus)
had grown to lengths of more than 15 m, and represented a group of marine carnivores that
were as dominant in their environment as Tyrannosaurus rex was on land. Unlike T-rex,
these giant mosasaurs were more widely distributed geographically and more numerous.
During Maastrichtian time, mosasaurs were diversifying rapidly and entering many niches
left vacant by the demise of other marine reptiles, and beginning to enter freshwater
environments. From their fossil record, it is evident that mosasaurs were a highly
successful group that became the apex predators of the Earth’s oceans rather suddenly
during the Late Cretaceous. For all their success, however, they may have become too
specialized and became extinct at or near the end of the Cretaceous, most likely due to a
collapse of the marine ecosystem.
The first mosasauroids appear in the fossil record during Cenomanian time in Europe and
the Western Interior Sea. Small terrestrial lizards considered to be ancestral to
mosasaurs (aigialosaurs and coniasaurs) have been found in the Adriatic region of
southeastern Europe and in North America. Carroll and Debraga (1992) reported three
mosasaur-like aigialosaur specimens from Cenomanian-Turonian (93 mya) deposits in
Yugoslavia, and Bell and Polcyn (1996) documented the distribution of coniasaurs in the
Western Interior Sea. Russell (1967) proposed two stem groups of aigialosaurids that were
“Clidastes-like” (including Mosasaurus) and “Platecarpus-like”
(including Tylosaurus). Martin and Stewart (1977), Bell (1995), and Bell and
VonLoh (1998) documented mosasauroid and early mosasaur remains from Kansas, South Dakota
and Texas. While it is likely that the first mosasaurs evolved from several different
aigialosaurid lineages, the geographic origin(s) of the earliest mosasaurs is still
uncertain. Their closest modern relatives are probably monitor lizards (varanids) like the
Komodo dragon and quite possibly snakes (Caldwell, 1999), although the exact relationships
are currently a matter of debate among mosasaur workers. Wherever they may have first
entered the sea, it appears that they were able to spread rapidly around the world by
migrating via shallower coastal waters. Similar species found as far apart as North
America and New Zealand suggest that the initial spread of mosasaurs occurred rapidly,
while other, highly derived species found in California and Africa provide evidence of the
relatively rapid diversification of isolated populations.
One of the issues related to the study of North American mosasaurs since 1868 has been the
sheer number of specimens in collections, and the tendency of earlier workers to name new
species from non-diagnostic material. Cope (1871) noted that a total of 6 species had been
discovered in the “cretaceous beds of Kansas.” The following year, Cope (1872)
reported 17 species from the Kansas chalk and a grand total of 42 species from Kansas,
Alabama and New Jersey. Marsh (1880) noted that the “Museum of Yale College contains
remains of not less than 1,400 distinct individuals.” Williston
(1891) observed that there had been “twice too many generic names given; so, too,
it is pretty evident that there is an even greater number of synonyms among the specific
names.” Many of the early Kansas specimens had been collected by Professor B. F. Mudge, and by Williston (1898, p. 200) himself,
who said “I have seen altogether not far from 2000 specimens of Mosasaurs, and have
collected with my own hands not less than 400.” Williston (ibid., p. 169-170) also
wrote that the “determination of the species described by early authors [mostly E. D.
Cope and O. C. Marsh] is in large part clearly impossible in the absence of the type
specimens” and “four-fifths of all described species must be abandoned.”
Since Williston’s time, the number of mosasaur genera and species has grown at a
slower, more measured pace.
In his study of the systematics and morphology of mosasaurs, Russell (1967, p. 121)
indicated that a major problem “was the proper application of 86 described species
names of American mosasaurs to the hundreds of fragmentary to nearly complete skeletons in
the collections of the American Museum and the Peabody Museum at Yale.” Not only had
Marsh and Cope named new species of mosasaurs from fragmentary material that would be
considered non-diagnostic today, they had neglected to properly curate many of their
“type specimens.” Russell (ibid.) reduced the number of genera and species of
mosasaurs to more realistic levels. However, during the last two decades, and especially
the last 10 years, discoveries of new material in various parts of the world have added
greatly to our knowledge of mosasaurs, and to the number of valid species that are now
recognized.
DISCUSSION
Although the pre-Coniacian remains of mosasaurs are rare, and usually
fragmentary, two new, relatively complete specimens are currently being described from
Texas by Bell and Polcyn (2005, in press) and Polcyn and Bell (2005, in press). It does
appear that the divergence between the Clidastes-like and Platecarpus-like
mosasaurs had occurred prior to the Turonian (Bell, 1995). Martin and Stewart (1977)
described two sets of vertebrae and a jaw fragment from the middle Turonian Fairport Chalk
Member of the Carlile Shale Formation in Kansas and noted their affinities with Clidastes.
Another skull element (a Platecarpus-like frontal; KUVP 97200; Bell, pers. comm., 2004)
from the same strata in Ellis County, Kansas is also in the University of Kansas
collection. Lingham-Soliar (1994) reviewed mosasaur remains from the Upper Turonian of
Angola in western Africa. Bell (1995) and Bell and VonLoh (1998) reported on fragmentary
specimens of mosasauroids from the Boquillas Formation of western Texas and the Greenhorn
Formation (Early Turonian) of South Dakota. A more detailed discussion of the
stratigraphic occurrence of mosasaurs and their discovery worldwide is provided by Bell
(1997). The first (and earliest) Tylosaurus
remains from Kansas have recently been described from the Lower Campanian Fort Hays
Limestone (Everhart, (2005b). Tylosaurus is certainly the earliest genus of large
mosasaurs.
The rise of mosasaurs can be visualized as occurring in three distinct waves (Table 2),
each of which radiated outward from their point of origin. By middle Coniacian time (87
mya), the "first wave" of mosasaurs (Tylosaurus, Platecarpus,
and Clidastes) was well established in the Western Interior Sea that covered
Kansas and most of the Midwest portion of North America (Williston, 1898; Russell, 1967;
Everhart 2001). As mosasaurs continued to evolve, growing larger and diversifying rapidly,
a "second wave" of genera and species, including Hainosaurus, Plioplatecarpus,
Mosasaurus, Globidens, and Prognathodon appeared near the
beginning of the Campanian (83.5 mya). Following a possible near-extinction event near the
middle of the Campanian reported by Lindgren (2004), mosasaurs rebounded, and a
"third wave" was just getting underway during the final years of the Cretaceous,
shortly before their final extinction.
| Table 2. Approximate age of occurrence of the
various genera of mosasaurs in the Upper Cretaceous, Western Interior Sea of North America
and elsewhere around the world. |
| Age |
Time |
Span |
Remarks |
| Maastrichtian |
71.3-65.4 MYA |
5.9 MY |
Greatest diversity and
distribution; invasion of freshwater habitats. Beginning of "Third Wave"
mosasaurs. |
| Campanian |
83.5-71.3 MYA |
12.2 MY |
"Second Wave"
mosasaurs; Hainosaurus, Mosasaurus, Globidens, Plioplatecarpus,
Prognathodon, Halisaurus |
| Santonian |
85.8-83.5 MYA |
2.3 MY |
Mosasaurs get much larger;
worldwide distribution |
| Coniacian |
89.0-85.8 MYA |
3.2 MY |
"First Wave"
mosasaurs; Tylosaurus, Platecarpus and Clidastes |
| Turonian |
93.5-89.0 MYA |
4.5 MY |
Earliest mosasaurs;
Precursors of Clidastes, Tylosaurus, and Platecarpus |
| Cenomanian |
99.0-93.5 MYA |
5.5 MY |
Ancestral mosasaurs
(Aigialosaurs?) return to the ocean |
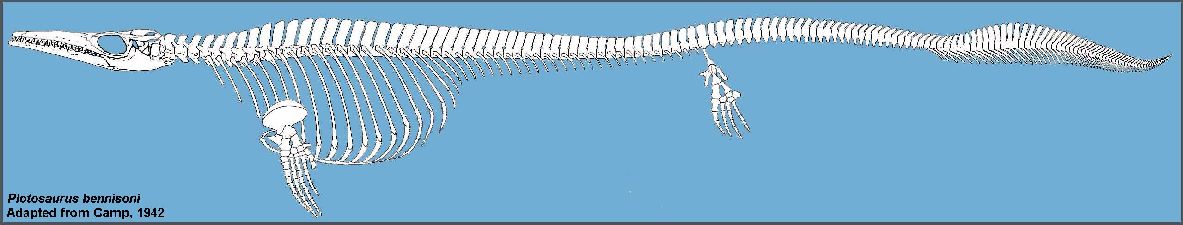
Camp (1942) reported on two highly derived species of mosasaurs (Plotosaurus and Plesiotylosaurus) from
the Maastrichtian age Moreno Formation of California that are quite unlike any other known
species and indicative of an isolated population. Modifications to the skull, paddles and
tail of Plotosaurus give it a decidedly ichthyosaurian appearance, and hint at a
life style or habitat preferences that differ from most other mosasaurs (See Lindgren,
Caldwell and Jagt (2008) for a more recent analysis). The crocodile-like Goronyosaurus
nigeriensis (Lingham-Soliar, 1999a) and ichthyosaur-like Pluridens walkeri (Lingham-Soliar,
1998) from the Maastrichtian of Africa also represent highly divergent lineages. The
durophagous mosasaurs (Globidens, Compressidens,
Carinodens and to some extent, Prognathodon) represent a group that
moved away from the “normal” mosasaur diet of fish and cephalopods (squid) and
evolved stronger, more rigid skulls and specialized teeth adapted for feeding upon
hard-shelled prey.
Size, body shape and integument
Aigialosaurs, the ancestors of mosasaurs, were small terrestrial reptiles that reached
lengths of about 1-2 m during Cenomanian time. By the early Coniacian, the largest genus
of mosasaurs, Tylosaurus, grew to lengths of about 7 m, with Platecarpus
and Clidastes being considerably smaller (Table 3). Following Cope’s Law,
mosasaurs generally increased in size through the last 25 million years of the Cretaceous,
with two species, Hainosaurus (a tylosaurine) and Mosasaurus reaching,
reaching nearly 17 m (Lingham-Soliar, 1999b). As mosasaurs diversified, however, there
were a number of smaller, durophagous species (e. g., Carinodens) that were
occupying other ecological niches during Campanian and Maastrichtian time.
| Table 3. Increase in the length of adult
mosasaurs through time: |
More than anything else, the long, slender body shape and skeleton of a mosasaur resembled
that of a snake. However, mosasaurs retained their front and rear limbs, and the chest
region was noticeably expanded, a possible indication that, unlike snakes, they retained
two lungs. The head was conical in shape and was extremely long and narrow in some species
(e.g., Ectenosaurus clidastoides). While
their body shape might be considered relatively inefficient for high-speed swimming
compared with the models provided by ichthyosaurs and plesiosaurs, it appears likely that
mosasaurs were more of an ambush predator than a pursuit predator (Massare, 1988). In that
regard, the body shape, larger, more flexible skull, and swimming style evolved by
mosasaurs appears to have been a more successful strategy for competing in the marine
environment.
Although Marsh (1872) claimed to have discovered “dermal
scutes” on mosasaurs, they were re-examined by Williston
(1891) and found to be fragments of the thin sclerotic ring that covered much of the
outer surface of the eye of the mosasaur. Snow (1878), at the
University of Kansas, was the first to report on the dermal covering of mosasaurs, citing
as an example the remains of an estimated 8 m long Tylosaurus proriger specimen
(KUVP 1075) he had discovered in Gove County in 1877. Snow published a photograph showing
the impressions of about 3000 scales and noted (ibid., p. 56) that there were about 90
scales per inch, being somewhat smaller than those of a large rattlesnake (80 scales per
inch). The scales are diamond shaped (3.3 mm x 2.5 mm), with a raised ridge (carina) on
the long axis. Scales of a similar size and shape, but lacking the central ridge, are also
known from a specimen of Ectenosaurus clidastoides (FHSM VP-401) in the
collection of the Sternberg Museum of Natural History. Numerous patches of small,
diamond-shaped scales are visible on the bones on the underside of a Platecarpus
tympaniticus specimen from Kansas reported by Geist et al. (2002). All of these
specimens are of late Santonian to early Campanian age, and lived some 20-18 million years
before the end of the Cretaceous. Skin impressions, however, are unknown from younger
specimens of mosasaurs. Given the apparent smooth integument of ichthyosaurs and
plesiosaurs, it appears likely that mosasaurs may have also lost their scales as they
evolved further. (NOTE: Lindgren, et al., 2009 demonstrated
that Plotosaurus bennisoni from the Middle Maastrichtian of California had scales)
Locomotion
Early mosasaurs were somewhat more conservative in the evolution of their body plan than
the highly derived ichthyosaurs and plesiosaurs. Although they used the same side-to-side
movement of their tail as the ichthyosaurs (compared to the wing-shaped paddles and
underwater flight of plesiosaurs), they did not develop the lobed, semi-lunate tail. In
overall appearance, they resembled a crocodile with paddles instead of legs and feet. It
appears likely that most mosasaurs used their limbs for steering and attitude control,
although the flexible, highly cartilaginous paddles of Tylosaurus
may have had a slightly different functionality compared to the much more solid limb bones
of Clidastes and Mosasaurus (Figure 1). In most early mosasaurs, the
widely-spread digits were loosely joined by a webbing to form a flexible paddle, while in
later genera (e. g., Plioplatecarpus and Plotosaurus) the digits were
arranged tightly together to form a stiffer, wing-like structure. These highly derived
paddles may have been more useful for propulsion in other than open-water environments
(Lingham-Soliar, 1992: for a counter argument, see Nicholls and Godfrey, 1994). In either
case, mosasaur limbs and limb girdles were rather quickly modified to the point that they
would no longer support the weight of the animal out of the water. Based on the discovery
of embryos in an aigialosaur (Caldwell and Lee, 2001) from the Cenomanian-Turonian of
Slovenia, it is likely that live birth had evolved in the early mosasauroids at some
before they returned completely to the sea. Freed from the necessity of laying their eggs
on land, mosasaurs were probably completely marine animals by the early Turonian.
The muscular tail of mosasaurs was flattened laterally and was used to propel the animal
through the water with a sinuous, side-to-side movement. Although somewhat shorter
proportionally than in most terrestrial lizards, the tail
of mosasaurs was still quite long, up to 42% of body length in Clidastes liodontus
and 52% in Tylosaurus proriger (Russell, 1967). In some genera (i.e. Clidastes
and Plotosaurus), the surface area of the tail
was increased by lengthening the neural spines of the vertebrae. This may have
occurred as a means of increasing thrust in compensation for the relatively shorter
length. Unlike modern sea snakes that undulate their entire body while swimming, the
pre-caudal vertebrae of mosasaurs were relatively inflexible laterally and provided a
stable base for the muscular tail. The head and neck were probably held in line with the
dorsal vertebrae while swimming while the paddles were folded against the side of the
animal except to change directions. Although capable of swimming long distances and
remaining at sea indefinitely, mosasaurs probably were not as fast as ichthyosaurs and the
polycotylid plesiosaurs (Massare, 1988). Where they did excel apparently, was as ambush
predators, using surprise and rapid acceleration to overtake and capture their prey
(Massare, 1987). The fact that thousands of mosasaur specimens have been collected from
the Niobrara Chalk in western Kansas, a locality that would have been hundreds of km from
the nearest land from Coniacian through early Campanian time, indicates that they were as
well adapted to life in the sea as modern sea mammals. Jacobs, et al. (2004) reported that
modern sea snakes and marine iguanas are limited to habitats where the surface water
temperature ranges between 20º and 35º C. Even so, iguanas in the Galapagos Islands have
to sun themselves for extended periods to increase their body temperature between feeding
forays. This raises questions regarding changes in the ancestral reptilian metabolism and
circulatory system of mosasaurs that would both support swimming over long periods of time
and the continuous loss of body heat to the surrounding seawater. Additional studies are
necessary to address these issues. (Added June 11, 2010 -
See Bernard, et al. 2010 in Science for new information regarding the body
temperature of marine reptiles. See also Motani 2010 for comments)
Feeding adaptations
Marine creatures face different problems than terrestrial forms in acquiring and
swallowing prey because of the weightless, three-dimensional environment in which they
live. Captured prey, if released, can float away, sink or be taken by competitors. As land
dwelling reptiles that returned to the sea, mosasaurs had to adapt feeding strategies that
addressed these issues. In the case of most of the ichthyosaurs and plesiosaurs, the
solution was simply feeding on small fishes and cephalopods that could be captured and
swallowed intact. While the body plans of the fish-like ichthyosaurs and highly
streamlined plesiosaurs appear to be advantageous to capturing prey, both of these groups
also had relatively small, rigid skulls that limited the maximum size of the prey that
could be swallowed. Massare (1988) considered them to be pursuit predators, depending on
their speed to chase down and acquire prey. As a result, their feeding strategy required a
major expenditure of energy to catch large numbers of small fish and cephalopods. In the
case of ichthyosaurs, and possibly plesiosaurs, their eventual extinction may have been
related to the evolution of faster fishes and the competition from larger teleosts for the
same prey.
Mosasaurs, on the other hand, retained a relatively large skull that was 10-14% of their
total body length (Russell, 1967; Everhart, 2001). In addition, the skull in the earlier
genera (Tylosaurus, Platecarpus and Clidastes) was highly
kinetic (Russell, 1967), an adaptation seen in modern snakes that made the skull of these
animals flexible enough to swallow much larger prey. Other feeding adaptations in
mosasaurs included: a mobile quadrate that provided additional fore and aft movement to
the lower jaw; an intermandibular hinge that allowed the lower jaws to bow outward as the
prey was pulled into the mouth; pterygoid teeth that kept the prey from escaping as the
lower jaw disengaged and moved forward; and a symphysial hinge between the tips of the
lower jaws that allowed some degree of independent movement (Figure 2). The similarities
between the jaw mechanics of mosasaurs and snakes are discussed in detail by Lee, et al.
(1999).
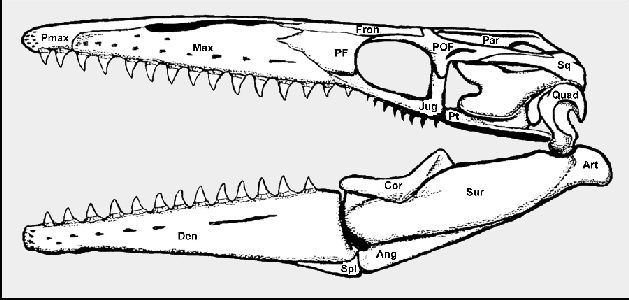 |
Figure 2. Tylosaurus kansasensis:
The skull of a new (Everhart, 2005a) mosasaur species from the Niobrara Chalk of western
Kansas. (Ang – angular; Art – articular; Cor – Coronoid; Den –
Dentary; Fron – Frontal; Jug – Jugal; Max – Maxilla; Par – Parietal;
PF – Prefrontal; POF – Postorbitalfrontal; Pt – Pterygoid; Quad –
Quadrate; Spl – Splenial; Sq – Squamosal; Sur – Surangular)
|
Cope (1872) was one of the first to describe the
feeding mechanism in mosasaurs: “They were furnished, like snakes, with four rows of
formidable teeth on the roof of the mouth. Though these were not designed for mastication,
and, without paws for grasping, could have been little used for cutting, as weapons for
seizing their prey they were very formidable. And here we have to consider a peculiarity
of these creatures, in which they are unique among animals. Swallowing their prey entire
like snakes, they were without that wonderful expandability of throat due in the latter to
an arrangement of levers supporting the lower jaw. Instead of this each half of that jaw
was articulated or jointed at a point nearly midway between the ear and the chin. This was
of the ball-and-socket type, and enabled the jaw to make an angle outward, and so widen by
much the space enclosed between it and its fellow. The arrangement maybe easily imitated
by directing the arms forward, with the elbows turned outward and the hands placed near
together. The ends of these bones were in the Pythonomorpha as independent as in the
serpents, being only bound by flexible ligaments. By turning the elbows outward and
bending them, the space between the arms becomes diamond-shaped and represents exactly the
expansion seen in these reptiles, to permit the passage of a large fish or other
body.”
The teeth of the earliest mosasaurs were simple cones that were slightly recurved
posteromedially. See Leidy (1858) for an early description of
the teeth of Mosasaurus. Smaller species, such as Clidastes and Platecarpus,
tended to retain slender, grabbing teeth for capturing small fish and cephalopods while
the teeth of the larger Tylosaurus were much more robust and were probably used
to seize and kill larger prey. Carina, if present, were small, and occasionally serrated.
Mosasaur teeth are generally indicative of prey preferences, with Tylosaurus and Prognathodon
being generalists that were capable of taking a variety of prey including large fish,
birds, other mosasaurs (Martin and Bjork, 1987), and small plesiosaurs (Sternberg, 1922; Everhart, 2004).
Only a few, poorly known species (e. g., Leiodon)
appear to have developed narrow teeth with efficient cutting edges to cut flesh or
dismember prey. At the other extreme, genera like Prognathodon evolved heavily
built conical teeth that were capable of crushing hard shelled prey such as ammonites and
turtles (Dollo, 1887; See bitten turtle shell fragment
here). First described by Gilmore (1912), Globidens
had a heavily built skull and rounded, ball-shaped teeth that were well adapted to feeding
on clams and other shellfish. A relatively complete, but as yet undescribed Globidens
specimen [NOTE: Globidens schurmanni was described by Martin (2007)] from the
Pierre Shale of South Dakota was found with fragments of several species of bivalves in
the abdominal area (Martin and Fox, 2004). The feeding mechanics of another, much smaller
mosasaur with an unusual assortment of grasping and
crushing teeth (Carinodens belgicus; Figure 3) were described by Schulp, et
al. (2004). Other species, such as Plotosaurus (Camp, 1942) and Pluridens
(Lingham-Soliar, 1998) appear to have evolved longer jaws that held a large number of
smaller teeth for more efficient feeding on smaller prey, similar to the feeding mechanism
of many ichthyosaurs.
 |
Figure 3. Restoration of Carinodens
belgicus by Wouter Verhesen: A small (2 m) mosasaur from the Maastrichtian of the
Netherlands with a highly derived dentition (Schulp, et al. 2004). Used with permission of
the artist and the Natuurhistorisch Museum Maastricht.
|
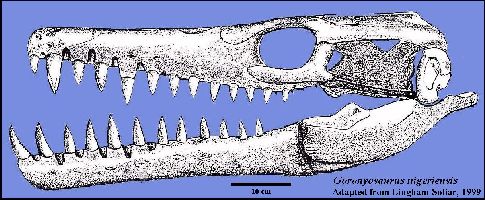 |
A highly derived African species (Goronyosaurus nigeriensis)
developed functional canine teeth and a heavily built skull that resembles that of a
crocodilian more than a mosasaur (Lingham-Soliar, 1999a). Remains of this Maastrichtian
species, as well as those of a Plioplatecarpus from Canada (Holmes, et al., 1999)
have been found in freshwater deposits, and may be evidence of competition between
mosasaurs and terrestrial crocodilians. LEFT: (Added figure): Reconstruction of the skull of Goronyosaurus
nigeriensis, based on the
holotype (BMNH R14153) and referred material. Adapted from Lingham-Soliar (1999a) (Scale =
10 cm). Another figure from Lingham-Soliar
(2002) is HERE: |
Live birth
It has been known for more than a century that ichthyosaurs gave live birth to their
young. An as yet undescribed polycotylid plesiosaur specimen from the Pierre Shale of
western Kansas shows indications of young within the body of an adult (Rothschild and
Martin, 1993). Although Williston (1898) and Russell (1967) argued against live birth in
mosasaurs, they did not cite evidence of nesting sites or protected areas where smaller
mosasaurs had been found. More recently, Bell, et. al. (1996) and Bell and Sheldon (2004)
reported the discovery of a mother mosasaur with skeletal elements of at least four babies
in her abdomen. Caldwell and Lee (2001) described a mosasaurid specimen from southeastern
Europe that contained embryonic material. Sheldon (1990) and (Everhart, 2002) noted the
presence of many specimens of small mosasaurs from the Smoky Hill Chalk, an indication of
mosasaurs were giving birth in mid-ocean. It is likely that the reproductive strategy of
mosasaurs involved the investment of the mother’s resources in a relatively small
number of larger, well-developed babies instead of laying a large number of eggs like
marine turtles or crocodiles. From the remains that have been found, it appears that baby
mosasaurs were between 1 and 2 m in length at birth (Everhart, 2002; Bell and Sheldon,
2004). In the case of the Niobrara Chalk (Coniacian through lower Campanian) of western
Kansas, the remains of very young mosasaurs were found in an area that would have been
more than 300 km from the nearest coast on the eastern edge of the Western Interior Sea.
While it has been speculated that there may have something similar to a kelp forest or
seaweed mat to provide shelter for small animals but no evidence for such a scenario has
been found. Even with larger sizes at birth, the survival rate of young mosasaurs was
probably low in an environment shared with large sharks, giant teleosts and other mosasaurs. However, the fossil record
indicates that enough individuals reached reproductive age to maintain population growth
over a long span of time.
Diversity and Distribution
As noted earlier, mosasaurs spread rapidly around the world during the later stages of the
Cretaceous, quickly becoming the apex predator of the Earth’s oceans and occupying
various other niches left vacant by the extinction of the ichthyosaurs, the reduction in
numbers of plesiosaurs, and possibly even the extinction of large, pelagic shark species
like Cretoxyrhina mantelli. While Williston (1898) and Russell (1967) reduced the
inflated number of species reported by Cope and Marsh, the number of recognized mosasaur
species continues to grow as new specimens are found and new localities are explored
(Table 1). Lingham-Soliar (1999b) estimated that there were about 20 genera and 45 species
of mosasaurs living at the end of the Cretaceous. Based on recent discoveries, it is
likely that those numbers may be underestimated by a factor of 2 or more. Mosasaurs appear
to have spread from the Western Interior Sea north and west along the rim of the western
Pacific, with similar genera showing up in Japan and across the equator in Australia and
New Zealand (Figure 4). Movement probably occurred in both directions across a much
narrower northern Atlantic between the east coast of North America and Europe. Similar
migrations appear to have occurred through the Tethys Ocean over submerged portions of
Europe and Africa, and into the Middle East. A new species of tylosaurine mosasaur (Lakumasaurus
antarcticus) discovered in upper Campanian – lower Maastrichtian deposits on an
island off the coast of Antarctica (Novas, et al., 2002) provides additional data on the
worldwide distribution of this genus. The presence of Mosasaurus
hobetsuensis in Japan (Suzuki, 1985b) and a recent discovery of a mosasaur much
like Mosasaurus hoffmanni in Turkey (Bardet and Tunoglu, 2002) indicates that,
like tylosaurines, this genus was also living in many places around the world.
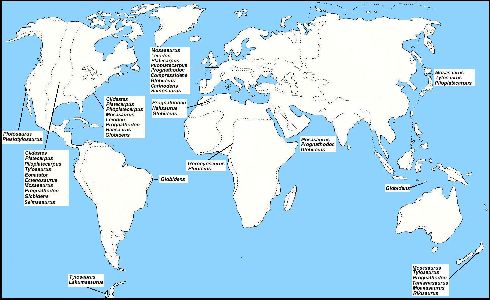 |
Figure 4. A generalized map of the Earth
showing the approximate locations of known mosasaur genera during Campanian-Maastrichtian
time. The dashed lines indicate the probable extent of epicontinental seas at the same
time. (Adapted from Suzuki, 1985a). Click here for a more detailed map of the Western Interior Sea of North America.
|
It is likely that the success of mosasaurs as predators led to a population
explosion and created pressure to expand into new territories. In the case of two of the
largest and most ubiquitous genera, Tylosaurus and Mosasaurus, the
distances traveled would have been roughly half way around the world (roughly 20,000 km).
While this is a relatively large distance, an expanding population could easily move that
far in 4000 years at an average rate of 5 km per year. For an animal that was well adapted
to living completely at sea, it is more likely that mosasaurs spread around the world at a
much faster rate. Although the origin, timing and direction of these
“migrations” is still uncertain, relict populations of highly derived mosasaurs
such as those found in the Maastrichtian of California and Africa may eventually shed some
light on these issues. New discoveries continue to add both to the number of species that
are known and the extent to which the various genera dispersed across the oceans of the
Late Cretaceous.
CONCLUSION
During the last 25 million years of the Cretaceous, mosasaurs evolved relatively quickly
from small shore-dwelling lizards into the dominant marine predators in the oceans of the
Earth. Their adaptations to life in the ocean included a highly kinetic skull and jaws,
major modifications to the axial skeleton and limbs, changes in body shape and covering,
growth to very large size, and live birth. The evolution of mosasaurs can be considered as
a pre-Tertiary model of rapid adaptive radiation.
ACKNOWLEDGEMENTS
I thank Gorden Bell, Mike Polcyn and Dale Russell for our continuing discussions of all
things mosasaur, J.D. Stewart and Donald Hattin for their insights into the stratigraphy
and the appearance of mosasaurs in the Niobrara Chalk of western Kansas. I am also
grateful to Richard Zakrzewski (Sternberg Museum of Natural History), Larry Martin and
Desui Miao (University of Kansas Museum of Natural History), James Martin and Carrie
Herbel (Museum of Geology, South Dakota School of Mines and Technology), Earle Spamer and
Ted Daeschler (Academy of Natural Sciences of Philadelphia), Robert Purdy and Michael
Brett-Surman (United States National Museum), Anne Schulp (Natuurhistorisch Museum
Maastricht) and Kazuhiko Sakurai (Hobetsu Museum, Hobetsu, Hokkaido, Japan) for access to
specimens in their care. Yoshiyuki Usami (Kanagawa University, Yokohama, Japan)
facilitated my trip to the conference in Nagoya, Japan in 2002.
LITERATURE CITED
Bardet N., X. P. Suberbiola, and N-E Jalil. 2003. A new mosasauroid (Squamata) from the
Late Cretaceous (Turonian) of Morocco. Comptes Rendus Palevol 2(8): 607-616.
Bardet N., X. P. Suberbiola, M. Iarochene, F. Bouyahyaoui, B. Bouya, and M. Amaghzaz.
2005. A new species of Halisaurus from the Late Cretaceous phosphates of Morocco,
and the phylogenetical relationships of the Halisaurinae (Squamata: Mosasauridae).
Zoological Journal of the Linnean Society 143: 447-472.
Bardet, N. and C. Tunoglu. 2002. The first mosasaur (Squamata) from the Late Cretaceous of
Turkey. Journal of Vertebrate Paleontology 22(3): 712-715.
Bell, G. L., Jr. 1995. Middle Turonian (Cretaceous) mosasauroids from Big Bend National
Park, Texas. In Santucci, V. L. and L. McClelland, (Eds.), National Park Service
Paleontological Research, U. S. Department of the Interior Technical Report
NPS/NRPO/NRTR-95/16, p. 34-39.
Bell, G. L. Jr. 1997. Part IV: Mosasauridae - Introduction. pp. 281-292 In Callaway J. M.
and E. L. Nicholls, (eds.), Ancient Marine Reptiles, Academic Press, San Diego.
Bell, G. L. Jr. and M. J. Polcyn. 1996. Distribution of the lizard, Coniasaurus,
in the western interior Cretaceous seaway and its paleoecological implications. Geological
Society of America, Rocky Mountain Section Annual Meeting, Abstracts with Programs, 28(4):
2.
Bell, G. L. Jr. and M. J. Polcyn. 2004. Polyphyly of Mosasauridae: The growing body of
evidence. In Schulp, A. S. and John W. M. Jagt (eds.), Abstract book and field guide of
the First Mosasaur Meeting, Natuurhistorisch Museum Maastricht, the Netherlands, p. 15.
Bell, G. L. Jr. and M. J. Polcyn. 2005. Dallasaurus turneri, gen. et. sp. nov.
(Squamata; Mosasaurinae); A new primitive mosasauroid from the Turonian of Texas and
comment on the polyphyly of Mosasauridae. Netherlands Journal of Geosciences / Geologie en
Mijnbouw.
Bell, G. L. Jr., M. A. Sheldon, J. P. Lamb and J. E. Martin. 1996. The first direct
evidence of live birth in Mosasauridae (Squamata): Exceptional preservation in Cretaceous
Pierre Shale of South Dakota. Journal of Vertebrate Paleontology 16(Supplement to 3): 21A.
Bell, G. L. Jr., and A. M. Sheldon. 2004. A gravid mosasaur (Plioplatecarpus)
from South Dakota. In Schulp, A. S. and John W. M. Jagt (eds.), Abstract book and field
guide of the First Mosasaur Meeting, Natuurhistorisch Museum Maastricht, the Netherlands,
p. 16.
Bell, G. L. Jr., and J. P. VonLoh. 1998. New records of Turonian mosasauroids from the
western United States. Fossil vertebrates of the Niobrara Formation in South Dakota,
Dakoterra 5: 15-28.
Bernard, A., Lécuyer, C., Vincent, P., Amiot, R.,
Bardet, N., Buffetaut, E., Cuny, G., Fourel, F., Martineau, F., Mazin, J-M. and
Prieur, A. 2010. Regulation of body temperature by some Mesozoic marine
reptiles. Science 328:1379-1382.
Caldwell, M. W. 1999. Squamate phylogeny and the relationships of snakes and mosasaurids,
Zoological Journal of the Linnean Society 125: 115-147, 7 figs.
Caldwell, M. W. and M. S. Y. Lee. 2001. Live birth in Cretaceous marine lizards
(mosasauroids). Proceedings: Biological Sciences 268(1484): 2397-2401.
Camp, C. L. 1942. California Mosasaurs. University of California Press, 67 pages.
Camper, A. G. 1800. Sur les ossemens fossiles de la montagne de St. Pierre, à Maëstricht
(Lettre de A. G. Camper à G. Cuvier). Journal de Physique 51: 278-291, pls. 1-2.
Carroll, R. L. and M. Debraga. 1992. Aigialosaurs: Mid-Cretaceous varanid lizards. Journal
of Vertebrate Paleontology 12(1): 66-86.
Cope, E. D. 1871. On the fossil reptiles and fishes of the Cretaceous rocks of Kansas.
Preliminary Report of the United States Geological Survey of Wyoming and Portions of the
Contiguous Territories (Hayden), Part IV, Special Reports, 6: 385-424.
Cope, E. D. 1872. On the geology and paleontology of the
Cretaceous strata of Kansas. Preliminary Report of the United States Geological Survey
of Montana and Portions of the Adjacent Territories, Part III - Paleontology, pp. 318-349.
Christiansen, P. and N. Bonde. 2002. A new species of gigantic mosasaur from the Late
Cretaceous of Israel. Journal of Vertebrate Paleontology 22(3): 629-644.
Dollo, L. 1887. Le Hainosaure et les nouveaux vertébrés fossiles du Musée de Bruxelles.
Revue des Questions Scientifiques 21: 504-539; 22: 70-112.
Dortangs, R. W., A. S. Schulp, E. W. A. Mulder, J. W. M. Jagt, H. H. G. Peeters and D. Th.
de Graaf. 2002. A large new mosasaur from the Upper Cretaceous of the Netherlands.
Netherlands Journal of Geosciences / Geologie en Mijnbouw 81(1): 1-8.
Everhart, M. J. 2001. Revisions to the biostratigraphy of the
Mosasauridae (Squamata) in the Smoky Hill Chalk Member of the Niobrara Chalk (Late
Cretaceous) of Kansas. Kansas Academy of Science, Transactions 104(1-2): 56-75.
Everhart, M. J. 2002. Remains of immature mosasaurs (Squamata; Mosasauridae) from the
Niobrara Chalk (Late Cretaceous) argue against nearshore nurseries. Journal of Vertebrate
Paleontology 22(Supplement to 3): 52A.
Everhart, M. J. 2004. Plesiosaurs as the food of mosasaurs; new data on the stomach
contents of a Tylosaurus proriger (Squamata; Mosasauridae) from the Niobrara
Formation of western Kansas. The Mosasaur 7: 41-46.
Everhart, M. J. 2005a. Tylosaurus kansasensis, a new species of tylosaurine
(Squamata, Mosasauridae) from the Niobrara Chalk of western Kansas, USA. Netherlands
Journal of Geosciences / Geologie en Mijnbouw 84(3), p. 231-240.
Everhart, M. J. 2005b. Earliest record of the genus Tylosaurus
(Squamata; Mosasauridae) from the Fort Hays Limestone (Lower Coniacian) of western Kansas.
Transactions 108 (3/4): 149-155.
Geist, N. R., Carpenter, S., and Stewart, J. D. 2002. Chemical and morphological analysis
of soft tissue preservation in a mosasaur. Journal of Vertebrate Paleontology
22(Supplement to 3): 57A.
Gilmore, C. W. 1912, A new mosasauroid reptile from the Cretaceous of Alabama. Proceedings
U.S. National Museum 40(1870): 489-484.
Holmes, R., M. W. Caldwell, and S. L. Cumbaa. 1999. A new specimen of Plioplatecarpus
(Mosasauridae) from the lower Maastrichtian of Alberta: Comments on allometry, functional
morphology, and paleoecology. Canadian Journal of Earth Science 36: 363-369.
Jacobs, L. L., M. J. Polcyn, K. Ferguson, C. Rennison and L. H. Taylor. 2004. Age,
environment, and habitat of dolichosaurs and primitive mosasauroids. In Schulp, A. S. and
John W. M. Jagt (eds.), Abstract book and field guide of the First Mosasaur Meeting,
Natuurhistorisch Museum Maastricht, the Netherlands, p. 51.
Kass, M. S. 1999. Prognathodon stadtmani (Mosasauridae): A new species from the
Mancos Shale (Lower Campanian) of western Colorado. In Gillette, D. D. (ed.), Vertebrate
Paleontology in Utah, Utah Geological Survey, Miscellaneous Publication 99-1: 275-294.
Konishi, T. and Caldwell, M. W. 2007. New specimens of Platecarpus planifrons
(Cope, 1874) (Squamata: Mosasauridae) and a revised taxonomy of the genus: Journal of
Vertebrate Paleontology 27(1): 59-72.
Lee, M. S., G. L. Bell, Jr. and M. W. Caldwell. 1999. The origin of snake feeding. Nature
400: 655-659.
Leidy, J. 1858. [Remarks on the teeth of Mosasaurus].
Proceedings of the Academy of Natural Sciences of Philadelphia 9:176.
Lindgren, J. 2004. An intercontinental mosasaur extinction event at the early/late
Campanian boundary. In Schulp, A. S. and John W. M. Jagt (eds.), Abstract book and field
guide of the First Mosasaur Meeting, Natuurhistorisch Museum Maastricht, the Netherlands,
p. 57-58.
Lindgren, J., Alwmark, C., Caldwell,
M.W. and Fiorillo, A.R. 2009. Skin of the
Cretaceous mosasaur Plotosaurus: implications for aquatic adaptations in giant
marine reptiles. The Royal Society,
Biological Letters, published online 8 April 2009. (Added 7/18/2009)
Lindgren, J., Caldwell, M.W. and Jagt, J.W.M. 2008. New data on the postcranial anatomy
of the California mosasaur Plotosaurus bennisoni (Camp, 1942) (Upper Cretaceous:
Maastrichtian), and the taxonomic status of P. tuckeri (Camp, 1942). Journal of
Vertebrate Paleontology 28(4):1043-1054.
Lingham-Soliar, T. 1992. A new mode of locomotion in mosasaurs: Subaqueous flying in Plioplatecarpus
marshii. Journal of Vertebrate Paleontology 12(4): 405-421.
Lingham-Soliar, T. 1994. The mosasaur "Angolasaurus" bocagei
(Reptilia: Mosasauridae) from the Turonian of Angola re-interpreted as the earliest member
of the genus Platecarpus, Paläontologische Zeitschrift 68(1/2): 267-282.
Lingham-Soliar, T. 1998. A new mosasaur Pluridens walkeri from the Upper
Cretaceous Maastrichtian of the Lullemmeden Basin, southwest Niger, Journal of Vertebrate
Paleontology, 18(4): 709-717.
Lingham-Soliar, T. 1999a. A functional analysis of the skull of Goronyosaurus
nigeriensis (Squamata: Mosasauridae) and its bearing on the predatory behavior and
evolution of this enigmatic taxon. Neues Jahrbuch fuer Geologie und Palaeontologie
Abhandlungen (Stuttgart). 213(3): 355-374.
Lingham-Soliar, T. 1999b. What happened 65 million years ago: The study of giant marine
reptiles throws new light on the last major mass extinction. Science Spectra 17: 20-29.
Lingham-Soliar, T. 2002. First occurrence of premaxillary caniniform teeth in the
Varanoidea: Presence in the extinct mosasaur Goronyosaurus (Squamata:
Mosasauridae) and its functional and paleoecological considerations. Lethaia, 35:187-190.
Marsh, O. C. 1872. Discovery of the dermal scutes of mosasaurid reptiles. American Journal
of Science, Series 3, 16: 290-292
Marsh, O. C. 1880. New characters of mosasauroid reptiles. American Journal of Science,
Series 3, 19: 83-87.
Martin, J. E. 2007. A new species of the durophagous
mosasaur, Globidens (Squamata: Mosasauridae) from the Late Cretaceous Pierre
Shale Group of central South Dakota, USA. Pages 167-176 in Martin, J. E. and Parris D. C.
(eds.), The Geology and Paleontology of the Late Cretaceous Marine Deposits of
the Dakotas. Geological Society of America, Special Paper 427.
Martin, J. E. and P. R. Bjork. 1987. Gastric residues associated with a
mosasaur from the late Cretaceous (Campanian) Pierre Shale in South Dakota. Dakoterra 3:
68-72.
Martin, J. E. and J. E. Fox. 2004. Molluscs in the stomach contents of Globidens,
a shell crushing mosasaur, from the Late Cretaceous Pierre Shale, Big Bend area of the
Missouri River, central South Dakota. Geological Society of America, 2004 Rocky Mountain
and Cordilleran Regions Joint Meeting, Abstracts with Programs, 36(4): 80.
Martin, L. D. and J. D. Stewart. 1977. The oldest (Turonian) mosasaurs from Kansas.
Journal of Paleontology 51(5): 973-975.
Massare, J. A. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles.
Journal of Vertebrate Paleontology 7(2): 121-137.
Massare, J. A. 1988. Swimming capabilities of Mesozoic marine reptiles: Implications for
method of predation. Paleobiology 14(2): 187-205.
Motani, R.
2010. Warm-blooded sea dragons? Science (Perspectives) 328:1361-1362.
Nicholls, E. L. and S. J. Godfrey. 1994. Subaqueous flight in mosasaurs - A discussion.
Journal of Vertebrate Paleontology 14(3): 450-452.
Nicholls, E. L., and D. Meckert. 2002. Marine reptiles from the Nanaimo Group (Upper
Cretaceous) of Vancouver Island. Canadian Journal of Earth Science 39(11): 1591-1603.
Novas, F. E., M. Fernández, Z. B. Gasparini, J. M. Lirio, H. J. Nuñez and P. Puerta.
2002. Lakumasaurus antarcticus, n. gen. et sp., a new mosasaur (Reptilia,
Squamata) from the Upper Cretaceous of Antarctica. Ameghiniana 39(2): 245-249.
Páramo-Fonseca, M. E. 2000. Yaguarasaurus columbianus (Reptilia, Mosasauridae),
a primitive mosasaur from the Turonian (Upper Cretaceous) of Columbia. Historical Biology
14: 121-131.
Polcyn, M. J. and G. L. Bell, Jr. 2005. A new mosasauroid, Russellosaurus coheni
nov. gen. et. sp., from the early Middle Turonian of Texas. Netherlands Journal of
Geosciences / Geologie en Mijnbouw 84(3), p. 321-333.
Polcyn, M.J. and Everhart, M.J. 2008. Description and
phylogenetic analysis of a new species of Selmasaurus (Mosasauridae:
Plioplatecarpinae) from the Niobrara Chalk of western Kansas. Proceedings of
the Second Mosasaur Meeting, Fort Hays Studies Special Issue 3, Fort Hays State
University, Hays, Kansas pp. 13-28.
Polcyn, M. J., E. Tchernov and L. L. Jacobs. 1999. The Cretaceous biogeography of the
eastern Mediterranean with a description of a new basal mosasauroid from Ein Yabrud,
Israel. Proceedings 2nd Gondwanan Dinosaur Symposium. 15: 259-290.
Polcyn, M. J., E. Tchernov and L. L. Jacobs. 2003. Haasiasaurus gen. nov., a new
generic name for the basal mosasauroid Haasia Polcyn et al., 1999. Journal of
Vertebrate Paleontology 23(2): 476.
Rothschild, B. M., and L. D. Martin. 1993. Paleopathology: Disease in the Fossil Record.
Boca Raton, Florida: CRC Press, 386 pp.
Russell, D. A. 1967. Systematics and morphology of American mosasaurs. Peabody Museum of
Natural History, Yale University Bulletin 23, 241 pp.
Sakurai, K., T. Chitoku, and N. Shibuya. 1999. A new species of Mosasaurus (Reptilia,
Mosasauridae) from Hobetsu, Hokkaido, Japan. The Bulletin of the Hobetsu Museum 15: 53-66.
Schulp, A.S, J. W. M. Jagt, and F. Fronken. 2004. New material of the mosasaur Carinodens
belgicus from the Upper Cretaceous of the Netherlands. Journal of Vertebrate
Paleontology 24(3): 744-747.
Schulp, A.S., Polcyn, M.J., Mateus, O., Jacobs,
L.L., and Morais, M.L. 2008. A new species of Prognathodon
(Squamata, Mosasauridae) from the Maastrichtian of Angola, and the affinities of the
mosasaur genus Liodon. Proceedings of the Second Mosasaur Meeting,
Fort Hays Studies Special Issue 3, Fort Hays State University, Hays, Kansas, pp.
1-12
Sheldon, M. A. 1990. Immature mosasaurs from the Niobrara: a sampling problem? Journal of
Vertebrate Paleontology Abstract 10(Supplement to 3): 42A.
Snow, F. H. 1878. On the dermal covering of a mosasauroid reptile.
Kansas Academy of Science, Transactions 6: 54-58, Figs. 1-2.
Sternberg, C. H. 1922. Explorations of the Permian of Texas and
the chalk of Kansas, 1918. Kansas Academy of Science, Transactions 30(1): 119-120.
Suzuki, S. 1985a. Upper Cretaceous mosasaur remains from southern part of central
Hokkaido, Japan; A preliminary report. Bulletin of the Hobetsu Museum 2: 31-42, 4 pl.
Suzuki, S. 1985b. A new species of Mosasaurus (Reptilia; Squamata) from the Upper
Cretaceous Hakobuchi Group in central Hokkaido, Japan. In Goto, et al. (Eds.), Evolution
and Adaptation of Marine Vertebrates, pp. 45-66. Association for Geological Collaboration
in Japan, Monograph 30.
Williston, S. W. 1891. Kansas mosasaurs. Science. 18(463):
345.
Williston, S. W. 1898. Mosasaurs. The University Geological Survey of Kansas, Part V, 4:
81-347, pls. 10-72.
Wright, K. R. and S. W. Shannon. 1988. Selmasaurus russelli, A new
plioplatecarpine mosasaur (Squamata, Mosasauridae) from Alabama. Journal of Vertebrate
Paleontology 8(1): 102-107.








